Acid rain facts for kids

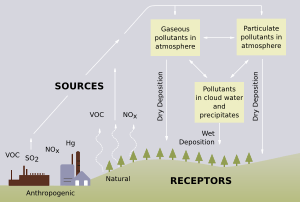
Acid rain is rain that is unusually acidic. The acids are made when certain gases are carried high into the sky and react with the water in the atmosphere.
Acid rains can be harmful to plants, animals, and humans.
Robert Angus Smith is credited with the term "acid rain." In 1852, he showed the relationship between acid rain and atmospheric pollution in Manchester.
Contents
Definition
"Acid rain" is a popular term referring to the deposition of a mixture from wet (rain, snow, sleet, fog, cloudwater, and dew) and dry (acidifying particles and gases) acidic components. Distilled water, once carbon dioxide is removed, has a neutral pH of 7. Liquids with a pH less than 7 are acidic, and those with a pH greater than 7 are alkaline. "Clean" or unpolluted rain has an acidic pH, but usually no lower than 5.7, because carbon dioxide and water in the air react together to form carbonic acid, a weak acid according to the following reaction:
- H
2O (l) + CO
2 (g) ⇌ H
2CO
3 (aq)
Carbonic acid then can ionize in water forming low concentrations of carbonate and hydronium ions:
- H
2O (l) + H
2CO
3 (aq) ⇌ HCO−
3 (aq) + H
3O+
(aq)
Unpolluted rain can also contain other chemicals which affect its pH (acidity level). A common example is nitric acid produced by electric discharge in the atmosphere such as lightning. Acid deposition as an environmental issue (discussed later in the article) would include additional acids other than H
2CO
3.
Occasional pH readings in rain and fog water of well below 2.4 have been reported in industrialized areas.
The main sources of the SO2 and NOx pollution that causes acid rain are burning fossil fuels to generate electricity and power internal combustion vehicles, to refine oil, and in industrial manufacturing and other processes.
Causes
Although some natural gases, such as carbon dioxide, and gases from volcanoes can cause acid rain, it is now believed that mankind causes most acid rain. When people began building and using factories and power stations, they burned coal or oil. These compounds have sulfur in them that releases into the air, mixes with other elements, and produces acid rain.
Since the 1970s, governments have tried to clean smoke from factories and power stations to keep too much sulfur from rising into the atmosphere. This has had good results, but it is expensive.
In 2001, Great Britain still produced about five million tonnes (metric tons) of these gases every year, while China produced 18 million tonnes. The United States produced more than 20 million tonnes, but in 2010 that number fell to 8.1 million.
Adverse effects
Effects on lake ecology

The pH level in lakes needs to be correct for fish to be able to survive, and acid rain can affect a lake's pH level. If there is too much acid in the water several things can happen:
- The enzymes that help fish larvae to hatch will not be produced.
- Aluminum travels more easily through water, causing fish to make more mucus around their gills. This makes it harder for fish to breathe.
- Phytoplankton will not grow well and the animals that feed on it would suffer.
Effects on soil biology
Acid rain can damage soil by killing microbes that are needed in the soil. The hydrogen ions of acid rain also allow toxins to move in the soil and take away nutrients that the soil needs. Forests usually are the home to fungi, but acid rain changes the soil, making it more filled with bacteria. Many trees in the forest depend on the fungi for water and minerals, while the fungi take carbohydrates from the trees. This symbiotic relationship, called mycorriza, can be destroyed by acids.
Other adverse effects

Acid rain affects both living and non-living things. The leaves and roots of trees are damaged, making them less able to make food (photosynthesis) and absorb nutrients. Toxic ions released due to acid rain are dangerous to humans. It is believed that copper (mobilized by acids) has caused outbreaks of diarrhea in young children. Statues and other ancient structures have also been harmed by acid rain.
Prevention methods
In the U.S., the method for keeping sulfur from escaping into the air from the smokestacks of factories is called Flue Gas Desulfurization (FGD). One popular example is a wet scrubber, which pulls the gases from the stack into a tower and cleans them using lime or limestone. This changes the form of the chemical into a calcium sulfate that can be taken out of the scrubber. Sometimes the sulfates are sold to chemical companies. Other times, they are put in a landfill.
Some people do not want power generation to be controlled because they feel that pollution is a natural consequence of factories and power stations. They believe that if the pollution is not handled properly, it could be dangerous. Others install pollution controls for the benefits that they will receive from them.
Interesting facts about acid rain
- Acid rain is a result of gases mixing with water high in the atmosphere.
- Sulfur dioxide is released into the air by machines, houses, and vehicles and is the main chemical that creates acid rain.
- Acid rain makes it more difficult for aquatic animals to survive.
- Acid rain can damage the soil and the plants that live in it.
- There are ways to prevent so much sulfur dioxide from escaping into the air. Many companies install pollution-controlling machinery in their factories.
Images for kids
-
Since 1998, Harvard University wraps some of the bronze and marble statues on its campus, such as this "Chinese stele", with waterproof covers every winter, in order to protect them from corrosion caused by acid rain and acid snow
See also
 In Spanish: Lluvia ácida para niños
In Spanish: Lluvia ácida para niños