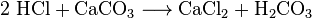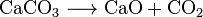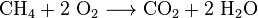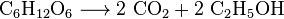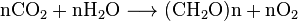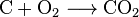Carbon dioxide facts for kids

Carbon dioxide (CO2) is a chemical compound. It is a gas at room temperature. It is made of one carbon and two oxygen atoms. People and animals release carbon dioxide when they breathe out. Also, every time something organic is burnt (or a fire is made), it makes carbon dioxide. Plants use carbon dioxide to make food. This process is called photosynthesis. The properties of carbon dioxide were studied by the Scottish scientist Joseph Black in the 1750s.
Carbon dioxide is a greenhouse gas. Greenhouse gases trap heat energy. Greenhouse gases change the climate and weather on our planet, Earth. This is called climate change. Greenhouse gases are a cause of global warming, the rise of Earth surface temperature.
Contents
Biological role
Carbon dioxide is an end product in organisms that obtain energy from breaking down sugars, fats and amino acids with oxygen as part of their metabolism. This is a process known as cellular respiration. This includes all plants, animals, many fungi and some bacteria. In higher animals, the carbon dioxide travels in the blood from the body's tissues to the lungs where it is breathed out. Plants take in carbon dioxide from the atmosphere to use in photosynthesis.
Dry ice
Dry ice, or solid carbon dioxide, is the solid state of CO2 gas below -109.3 °F (-78.5°C). Dry ice does not occur naturally on earth but is man made. It is colorless. People use dry ice to make things cold, and to make drinks fizzy, kill gophers, and freeze warts. The vapor of dry ice causes suffocation and eventually, death. Caution and professional assistance is recommended whenever dry ice is in use.
At usual pressure it will not melt from a solid to a liquid but instead changes directly from a solid to a gas. This is called sublimation. It will change directly from a solid to a gas at any temperature higher than extremely cold temperatures. Dry ice sublimates at normal air temperature. Dry ice exposed to normal air gives off carbon dioxide gas that has no color. Carbon dioxide can be liquified at pressure above 5.1 atmospheres.
Carbon dioxide gas that comes off of dry ice is so cold that when it mixes with air it cools the water vapour in the air to fog, which looks like a thick white smoke. It is often used in the theater to create the appearance of fog or smoke.
Isolation and production
Chemists can get carbon dioxide from cooling air. They call this air distillation. This method is inefficient because a large amount of air must be refrigerated to extract a small amount of CO2. Chemists can also use several different chemical reactions to separate carbon dioxide. Carbon dioxide is made in the reactions between most acids and most metal carbonates. For example, the reaction between hydrochloric acid and calcium carbonate (limestone or chalk) makes carbon dioxide:
The carbonic acid (H2CO3) then decomposes to water and CO2. Such reactions cause foaming or bubbling, or both. In industry, such reactions are used many times to neutralize waste acid streams.
Quicklime (CaO), a chemical that has widespread use, can be made heating limestone to about 850 °C. This reaction also makes CO2:
Carbon dioxide is also made in the combustion of all carbon-containing fuels, such as methane (natural gas), petroleum distillates (gasoline, diesel, kerosene, propane), coal or wood. In most cases, water is also released. As an example the chemical reaction between methane and oxygen is:
Carbon dioxide is made in steel mills. Iron is reduced from its oxides with coke in a blast furnace, producing pig iron and carbon dioxide:
Yeast metabolizes sugar to produce carbon dioxide and ethanol, also known as alcohol, in the production of wines, beers and other spirits, but also in the production of bioethanol:
All aerobic organisms produce CO2 when they oxidize carbohydrates, fatty acids, and proteins in the mitochondria of cells. The large number of reactions involved are exceedingly complex and not described easily. (They include cellular respiration, anaerobic respiration and photosynthesis). Photoautotrophs (i.e. plants, cyanobacteria) use another reaction: Plants absorb CO2 from the air, and, together with water, react it to form carbohydrates:
Carbon dioxide is soluble in water, in which it spontaneously interconverts between CO2 and H2CO3 (carbonic acid). The relative concentrations of CO2, H2CO3, and the deprotonated forms HCO−
3 (bicarbonate) and CO2−
3(carbonate) depend on the acidity (pH). In neutral or slightly alkaline water (pH > 6.5), the bicarbonate form predominates (>50%) becoming the most prevalent (>95%) at the pH of seawater, while in very alkaline water (pH > 10.4) the predominant (>50%) form is carbonate. The bicarbonate and carbonate forms are very soluble. So, air-equilibrated ocean water (mildly alkaline with typical pH = 8.2–8.5) contains about 120 mg of bicarbonate per liter.
Industrial production
Industrial carbon dioxide is produced mainly from six processes:
- By capturing natural carbon dioxide springs where it is produced by the action of acidified water on limestone or dolomite.
- As a by-product of hydrogen production plants, where methane is converted to CO2;
- From combustion of fossil fuels or wood;
- As a by-product of fermentation of sugar in the brewing of beer, whisky and other alcoholic beverages;
- From thermal decomposition of limestone, CaCO3, in the making of lime (Calcium oxide, CaO);
Chemical reaction
Carbon dioxide can be created with a simple chemical reaction:
Toxicity
Carbon dioxide content in fresh air varies between 0.036% (360 ppm) and 0.041% (412 ppm), depending on the location.
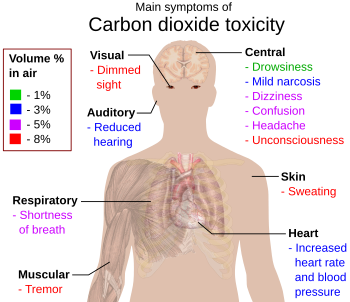
CO2 is not classified as toxic or harmful. In concentrations up to 1% (10,000 ppm), it will make some people feel drowsy and give the lungs a stuffy feeling. Concentrations of 7% to 10% (70,000 to 100,000 ppm) may cause suffocation, even in the presence of sufficient oxygen, manifesting as dizziness, headache, visual and hearing dysfunction, and unconsciousness within a few minutes to an hour.
Because it is heavier than air, in locations where the gas seeps from the ground (due to sub-surface volcanic or geothermal activity) in relatively high concentrations, without the dispersing effects of wind, it can collect in sheltered/pocketed locations below average ground level, causing animals located therein to be suffocated. Carrion feeders attracted to the carcasses are then also killed.

There are few studies of the health effects of long-term continuous CO2 exposure on humans and animals at levels below 1%. Occupational CO2 exposure limits have been set in the United States at 0.5% (5000 ppm) for an eight-hour period. At this CO2 concentration, International Space Station crew experienced headaches, lethargy, mental slowness, emotional irritation, and sleep disruption. Studies in animals at 0.5% CO2 have demonstrated kidney calcification and bone loss after eight weeks of exposure. A study of humans exposed in 2.5 hour sessions demonstrated significant negative effects on cognitive abilities at concentrations as low as 0.1% (1000 ppm) CO2 likely due to CO2 induced increases in cerebral blood flow. Another study observed a decline in basic activity level and information usage at 1000 ppm, when compared to 500 ppm.
Poor ventilation is one of the main causes of excessive CO2 concentrations in closed spaces, leading to poor indoor air quality.
Human physiology
Content
| Blood compartment | (kPa) | (mm Hg) | |
|---|---|---|---|
| Venous blood carbon dioxide | 5.5–6.8 | 41–51 | 41–51 |
| Alveolar pulmonary gas pressures |
4.8 | 36 | 36 |
| Arterial blood carbon dioxide | 4.7–6.0 | 35–45 | 35–45 |
The body produces approximately 2.3 pounds (1.0 kg) of carbon dioxide per day per person, containing 0.63 pounds (290 g) of carbon. In humans, this carbon dioxide is carried through the venous system and is breathed out through the lungs, resulting in lower concentrations in the arteries. The carbon dioxide content of the blood is often given as the partial pressure, which is the pressure which carbon dioxide would have had if it alone occupied the volume. In humans, the blood carbon dioxide contents is shown in the adjacent table.
Transport in the blood
CO2 is carried in blood in three different ways. (Exact percentages vary between arterial and venous blood).
- Majority (about 70% to 80%) is converted to bicarbonate ions HCO−
3 by the enzyme carbonic anhydrase in the red blood cells, by the reaction:
- CO
2 + H
2O → H
2CO
3 → H+
+ HCO−
3
- 5–10% is dissolved in blood plasma
- 5–10% is bound to hemoglobin as carbamino compounds
Hemoglobin, the main oxygen-carrying molecule in red blood cells, carries both oxygen and carbon dioxide. However, the CO2 bound to hemoglobin does not bind to the same site as oxygen. Instead, it combines with the N-terminal groups on the four globin chains. However, because of allosteric effects on the hemoglobin molecule, the binding of CO2 decreases the amount of oxygen that is bound for a given partial pressure of oxygen. This is known as the Haldane Effect, and is important in the transport of carbon dioxide from the tissues to the lungs. Conversely, a rise in the partial pressure of CO2 or a lower pH will cause offloading of oxygen from hemoglobin, which is known as the Bohr effect.
Regulation of respiration
Carbon dioxide is one of the mediators of local autoregulation of blood supply. If its concentration is high, the capillaries expand to allow a greater blood flow to that tissue.
Bicarbonate ions are crucial for regulating blood pH. A person's breathing rate influences the level of CO2 in their blood. Breathing that is too slow or shallow causes respiratory acidosis, while breathing that is too rapid leads to hyperventilation, which can cause respiratory alkalosis.
Although the body requires oxygen for metabolism, low oxygen levels normally do not stimulate breathing. Rather, breathing is stimulated by higher carbon dioxide levels. As a result, breathing low-pressure air or a gas mixture with no oxygen at all (such as pure nitrogen) can lead to loss of consciousness without ever experiencing air hunger. This is especially perilous for high-altitude fighter pilots. It is also why flight attendants instruct passengers, in case of loss of cabin pressure, to apply the oxygen mask to themselves first before helping others; otherwise, one risks losing consciousness.
The respiratory centers try to maintain an arterial CO2 pressure of 40 mmHg. With intentional hyperventilation, the CO2 content of arterial blood may be lowered to 10–20 mmHg (the oxygen content of the blood is little affected), and the respiratory drive is diminished. This is why one can hold one's breath longer after hyperventilating than without hyperventilating. This carries the risk that unconsciousness may result before the need to breathe becomes overwhelming, which is why hyperventilation is particularly dangerous before free diving.
Images for kids
See also
 In Spanish: Dióxido de carbono para niños
In Spanish: Dióxido de carbono para niños