Quick facts for kids
Curium, 96Cm
| Curium |
| Pronunciation |
(KEWR-ee-əm) |
| Appearance |
silvery metallic, glows purple in the dark |
| Mass number |
247 |
| Curium in the periodic table |
|
|
| Atomic number (Z) |
96 |
| Group |
n/a |
| Period |
period 7 |
| Block |
f |
| Electron configuration |
[Rn] 5f7 6d1 7s2 |
| Electrons per shell |
2, 8, 18, 32, 25, 9, 2 |
| Physical properties |
| Phase at STP |
solid |
| Melting point |
1613 K (1340 °C, 2444 °F) |
| Boiling point |
3383 K (3110 °C, 5630 °F) |
| Density (near r.t.) |
13.51 g/cm3 |
| Heat of fusion |
13.85 kJ/mol |
Vapor pressure
| P (Pa) |
1 |
10 |
100 |
1 k |
10 k |
100 k |
| at T (K) |
1788 |
1982 |
|
|
|
|
|
| Atomic properties |
| Oxidation states |
+2, +3, +4, +5, +6, (an amphoteric oxide) |
| Electronegativity |
Pauling scale: 1.3 |
| Ionization energies |
|
| Atomic radius |
empirical: 174 pm |
| Covalent radius |
169±3 pm |
|
Spectral lines of curium |
| Other properties |
| Natural occurrence |
synthetic |
| Crystal structure |
double hexagonal close-packed (dhcp)
|
| Electrical resistivity |
1.25 µΩ⋅m |
| Magnetic ordering |
antiferromagnetic-paramagnetic transition at 52 K |
| CAS Number |
7440-51-9 |
| History |
| Naming |
named after Marie Skłodowska-Curie and Pierre Curie |
| Discovery |
Glenn T. Seaborg, Ralph A. James, Albert Ghiorso (1944) |
| Main isotopes of curium |
| Isotope |
Abundance |
Half-life (t1/2) |
Decay mode |
Product |
| 242Cm |
syn |
160 d |
SF |
– |
| α |
238Pu |
| 243Cm |
syn |
29.1 y |
α |
6.169 |
239Pu |
| ε |
0.009 |
243Am |
| SF |
– |
– |
| 244Cm |
syn |
18.1 y |
SF |
– |
| α |
240Pu |
| 245Cm |
syn |
8500 y |
SF |
– |
| α |
241Pu |
| 246Cm |
syn |
4730 y |
α |
242Pu |
| SF |
– |
| 247Cm |
syn |
1.56×107 y |
α |
243Pu |
| 248Cm |
syn |
3.40×105 y |
α |
244Pu |
| SF |
– |
| 250Cm |
syn |
9000 y |
SF |
– |
– |
| α |
5.169 |
246Pu |
| β− |
0.037 |
250Bk |
|
Curium is a chemical element. It is a radioactive metal. It has the chemical symbol Cm. It has the atomic number 96. In chemistry it is placed in a group of metal elements named the actinides. Curium is a transuranic element. It is a radioactive element that does not exist in nature. Curium has to be made in a lab. Curium has a silver color and it is made by bombarding a plutonium target with alpha particles (helium ions). Curium was named after Marie Curie and her husband Pierre.
Images for kids
-
-
The 60-inch (150 cm) cyclotron at the Lawrence Radiation Laboratory, University of California, Berkeley, in August 1939.
-
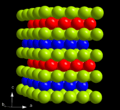
Double-hexagonal close packing with the layer sequence ABAC in the crystal structure of α-curium (A: green, B: blue, C: red)
-
Orange fluorescence of Cm3+ ions in a solution of tris(hydrotris)pyrazolylborato-Cm(III) complex, excited at 396.6 nm.
-
-
Several isotopes of curium were detected in the fallout from the Ivy Mike nuclear test.
-
Chromatographic elution curves revealing the similarity between Tb, Gd, Eu lanthanides and corresponding Bk, Cm, Am actinides.
-
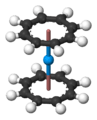
Predicted curocene structure
-
The radiation from curium is so strong that the metal glows purple in the dark.
-
Alpha-particle X-ray spectrometer of a Mars exploration rover
See also
 In Spanish: Curio para niños
In Spanish: Curio para niños
 Curium Facts for Kids
Curium Facts for Kids.
Kiddle Encyclopedia.
 In Spanish: Curio para niños
In Spanish: Curio para niños