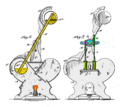Drinking bird facts for kids
Quick facts for kids Drinking bird |
|
|---|---|

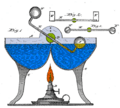
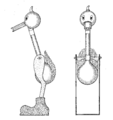
Drinking bird about to dip its beak in the water
|
|
| Classification | Heat engines |
| Application | Toy, Scientific demonstration |
| Fuel source | Heat transfer |
| Components | Bulbs, Tube, Axle, Support |
| Inventor | Miles V. Sullivan / Chinese Craftspeople |
| Invented | 1945 / much earlier than 1920 |
Drinking birds, also known as insatiable birdies, dunking birds, drinky birds, water birds or dipping birds, are toy heat engines that mimic the motions of a bird drinking from a water source. They are sometimes incorrectly considered examples of a perpetual motion device.
Contents
Construction and materials
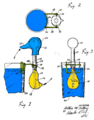
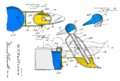
A drinking bird consists of two glass bulbs joined by a glass tube (the bird's neck). The tube extends nearly all the way into the bottom bulb, and attaches to the top bulb but does not extend into it. The space inside the bird contains a fluid, usually colored. The fluid is typically dichloromethane, also known as methylene chloride. Earlier versions contained trichlorofluoromethane. Miles V. Sullivan's 1945 patent suggested ether, alcohol, carbon tetrachloride, or chloroform.
Air is removed from the apparatus during manufacture, so the space inside the body is filled by vapor evaporated from the fluid. The upper bulb has a "beak" attached which, along with the head, is covered in a felt-like material. The bird is typically decorated with paper eyes, a plastic top hat, and one or more tail feathers. The whole setup pivots on an adjustable crosspiece attached to the neck.
Despite the drinking bird's appearance and classification as a toy there is a potential danger of thin shards of glass should the bulb break. Early models were often filled with highly flammable substances, though the fluid in later versions is nonflammable. Dichloromethane can irritate the skin on contact and the lungs if inhaled. It may be dangerous to people with pre-existing heart, liver, or nervous system conditions and is a suspected carcinogen.
Heat engine steps
The drinking bird is a heat engine that exploits a temperature difference to convert heat energy to a pressure difference within the device, and performs mechanical work. Like all heat engines, the drinking bird works through a thermodynamic cycle. The initial state of the system is a bird with a wet head oriented vertically.
The process operates as follows:
- The water evaporates from the felt on the head.
- Evaporation lowers the temperature of the glass head (heat of vaporization).
- The temperature decrease causes some of the dichloromethane vapor in the head to condense.
- The lower temperature and condensation together cause the pressure to drop in the head (governed by Equations of state).
- The higher vapor pressure in the warmer base pushes the liquid up the neck.
- As the liquid rises, the bird becomes top heavy and tips over.
- When the bird tips over, the bottom end of the neck tube rises above the surface of the liquid in the bottom bulb.
- A bubble of warm vapor rises up the tube through this gap, displacing liquid as it goes.
- Liquid flows back to the bottom bulb (the toy is designed so that when it has tipped over the neck's tilt allows this). Pressure equalizes between top and bottom bulbs.
- The weight of the liquid in the bottom bulb restores the bird to its vertical position.
- The liquid in the bottom bulb is heated by ambient air, which is at a temperature slightly higher than the temperature of the bird's head.
If a glass of water is placed so that the beak dips into it on its descent, the bird will continue to absorb water and the cycle will continue as long as there is enough water in the glass to keep the head wet. However, the bird will continue to dip even without a source of water, as long as the head is wet, or as long as a temperature differential is maintained between the head and body. This differential can be generated without evaporative cooling in the head; for instance, a heat source directed at the bottom bulb will create a pressure differential between top and bottom that will drive the engine. The ultimate source of energy is the temperature gradient between the toy's head and base; the toy is not a perpetual motion machine.
Physical and chemical principles
The drinking bird is an exhibition of several physical laws and is therefore a staple of basic chemistry and physics education. These include:
- The dichloromethane with a low boiling point (39.6 °C, 103.28 °F under standard pressure p
o= 105 Pa - as the drinking bird is first evacuated, partially filled and sealed, the pressure and thus the boiling point in the drinking bird will be different), gives the heat engine the ability to extract motion from low temperatures. The drinking bird is a heat engine that works at room temperature. - The combined gas law, which establishes a proportional relationship between temperature and pressure exerted by a gas in a constant volume.
- The ideal gas law, which establishes a proportional relationship between number of gas particles and pressure in a constant volume.
- The Maxwell–Boltzmann distribution, which establishes that molecules in a given space at a given temperature vary in energy level, and therefore can exist in multiple phases (solid/liquid/gas) at a single temperature.
- Heat of vaporization (or condensation), which establishes that substances absorb (or give off) heat when changing state at a constant temperature.
- Torque and center of mass.
- Capillary action of the wicking felt.
- Wet-bulb temperature: The temperature difference between the head and body depends on the relative humidity of the air.
By considering the difference between the wet and dry bulb temperatures, it is possible to develop a mathematical expression to calculate the maximum work that can be produced from a given amount of water "drunk". Such analysis is based on the definition of the Carnot heat engine efficiency and the psychrometric concepts.
Alternative design
In 2003 an alternative mechanism was devised by Nadine Abraham and Peter Palffy-Muhoray of Ohio, USA, that utilizes capillary action combined with evaporation to produce motion, but has no volatile working fluid. Their paper "A Dunking Bird of the Second Kind" , was submitted to the American Journal of Physics, and published in June 2004. It describes a mechanism which, while similar to the original drinking bird, operates without a temperature difference. Instead it utilizes a combination of capillary action, gravitational potential difference and the evaporation of water to power the device.
This bird works as follows: it is balanced such that, when dry, it tips into a head-down position. The bird is placed next to a water source such that this position brings its beak into contact with water. Water is then lifted into the beak by capillary action (the authors used a triangular sponge) and carried by capillary action past the fulcrum to a larger sponge reservoir which they fashioned to resemble wings. When enough water has been absorbed by the reservoir, the now-heavy bottom causes the bird to tip into a head-up position. With the beak out of the water, eventually enough water evaporates from the sponge that the original balance is restored and the head tips down again. Although a small drop in temperature may occur due to evaporative cooling, this does not contribute to the motion of the bird. The device operates relatively slowly with 7 hours 22 minutes being the average cycle time measured.
Images for kids
See also
 In Spanish: Pájaro bebedor para niños
In Spanish: Pájaro bebedor para niños