Image: Rutherford gold foil experiment results
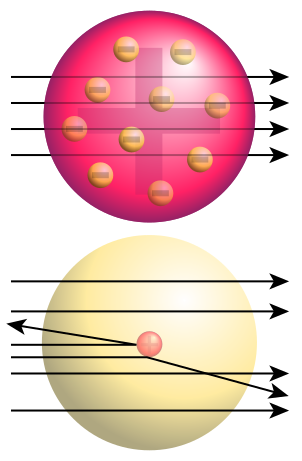
Description: Top: Expected results of Rutherford's gold foil experiment: alpha particles passing through the plum pudding model of the atom undisturbed. Bottom: Observed results: Some of the particles were deflected, and some by very large angles. Rutherford concluded that the positive charge of the atom must be concentrated into a very small location: the atomic nucleus.
Title: Rutherford gold foil experiment results
Credit: Own work
Author: Drawn by User:Fastfission in Illustrator and Inkscape. --Fastfission 15:04, 14 April 2008 (UTC)
Permission: If you want to credit someone, credit "Wikimedia Commons." Otherwise don't credit anyone, that's fine by me.
Usage Terms: Public domain
License: Public domain
Attribution Required?: No
Image usage
The following page links to this image:

