Oleuropein facts for kids
Quick facts for kids Oleuropein |
|
|---|---|
 |
|
|
Preferred IUPAC name
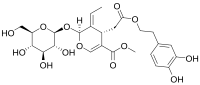
Methyl (2S,3E,4S)-4-{2-[2-(3,4-Dihydroxyphenyl)ethoxy]-2-oxoethyl}-3-ethylidene-2-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-2H-pyran-5-carboxylate
|
|
| Other names | 2-(3,4-Dihydroxyphenyl)ethyl [(2S,3E,4S)-3-ethylidene-2-(β-D-glucopyranosyloxy)-5-(methoxycarbonyl)-3,4-dihydro-2H-pyran-4-yl]acetate |
| Identifiers | |
| CAS number | |
| PubChem | |
| SMILES | O=C(OCCc1ccc(O)c(O)c1)C[C@H]2C(=C/C)\[C@@H](O\C=C2\C(=O)OC)O[C@@H]3O[C@@H]([C@@H](O)[C@H](O)[C@H]3O)CO |
|
InChI
InChI=1/C25H32O13/c1-3-13-14(9-19(29)35-7-6-12-4-5-16(27)17(28)8-12)15(23(33)34-2)11-36-24(13)38-25-22(32)21(31)20(30)18(10-26)37-25/h3-5,8,11,14,18,20-22,24-28,30-32H,6-7,9-10H2,1-2H3/b13-3+/t14-,18+,20+,21-,22+,24-,25-/m0/s1
|
|
| Properties | |
| Molecular formula | C25H32O13 |
| Molar mass | 540.44 g mol-1 |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
Oleuropein is a glycosylated seco-iridoid, a type of phenolic bitter compound found in green olive skin, flesh, seeds, and leaves. The term oleuropein is derived from the botanical name of the olive tree, Olea europaea.
Because of its bitter taste, oleuropein must be completely removed or decomposed to make olives edible. During processing of bitter and inedible green olives for consumption as table olives, oleuropein is removed from olives via a number of methods, including by immersion in lye.
Chemical treatment
Oleuropein is a derivative of elenolic acid linked to the orthodiphenol hydroxytyrosol by an ester bond and to a molecule of glucose by a glycosidic bond. When olives are immersed in a lye solution, the alkaline conditions lead to hydrolysis of the ester bond. The basic conditions also significantly increases the solubility of these derivatives, facilitating their release into the lye solution.
The high pH accelerates the oxidation of the phenolics, leading to blackness, as during their normal ripening, if the solution is oxygenated by air injection (alkaline oxidation of olives is also called the California process).
The lye solution is replaced several times until the bitter taste has dissipated. An alternative process uses amberlite macroporous resins to trap the oleuropein directly from the solution, reducing waste water while capturing the extracted molecules.
Enzymatic hydrolysis during the maturation of olives is also an important process for the decomposition of oleuropein and elimination of its bitter taste.
Green olive blackening
Green olives may be treated industrially with ferrous gluconate (0.4 wt. %) to change their color to black. Gluconate, an edible oxidation product of glucose, is used as non-toxic reactant to maintain Fe2+ in solution. When in contact with polyphenols, the ferrous ions form a black complex, giving the final color of the treated olives. Black olives treated with iron(II) gluconate are also depleted in hydroxytyrosol, as iron salts are catalysts for its oxidation.
Research
Oleuropein has been proposed as a proteasome activator.
See also
 In Spanish: Oleuropeína para niños
In Spanish: Oleuropeína para niños

