Tin facts for kids
 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tin | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Allotropes | silvery-white, β (beta); gray, α (alpha) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | silvery-white (beta, β) or gray (alpha, α) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight Ar, std(Sn) | 118.710(7) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tin in the periodic table | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic number (Z) | 50 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group | group 14 (carbon group) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Period | period 5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Block | p | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Kr] 4d10 5s2 5p2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 18, 4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase at STP | solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 505.08 K (231.93 °C, 449.47 °F) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | 2875 K (2602 °C, 4716 °F) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density (near r.t.) | white, β: 7.265 g/cm3 gray, α: 5.769 g/cm3 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| when liquid (at m.p.) | 6.99 g/cm3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | white, β: 7.03 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | white, β: 296.1 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar heat capacity | white, β: 27.112 J/(mol·K) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Vapor pressure
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | −4, −3, −2, −1, +1, +2, +3, +4 (an amphoteric oxide) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 1.96 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ionization energies |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius | empirical: 140 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 139±4 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Van der Waals radius | 217 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectral lines of tin | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Natural occurrence | primordial | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
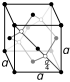
| Crystal structure | tetragonal
white (β) |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | face-centered diamond-cubic
gray (α) |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound thin rod | 2730 m/s (at r.t.) (rolled) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal expansion | 22.0 µm/(m⋅K) (at 25 °C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 66.8 W/(m⋅K) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrical resistivity | 115 nΩ⋅m (at 0 °C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Magnetic ordering | gray: diamagnetic white (β): paramagnetic |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar magnetic susceptibility | (white) +3.1·10−6 cm3/mol (298 K) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Young's modulus | 50 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Shear modulus | 18 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bulk modulus | 58 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Poisson ratio | 0.36 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Brinell hardness | 50–440 MPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Number | 7440-31-5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| History | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Discovery | around 3500 BC | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symbol | "Sn": from Latin stannum | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Main isotopes of tin | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Tin is a chemical element with symbol Sn (for Latin: stannum) and atomic number 50. It is in Group 14 on the periodic table. It is not radioactive.
Contents
Properties
Physical properties
Tin is a silver, somewhat soft metal. It is a post-transition metal. Its melting point is 231.93°C and its boiling point is 2602 °C. It can melt easily in a flame. It is malleable. It makes a crackling sound called tin cry when a piece of it is bent. Tin has more non-radioactive isotopes than any other element.
Tin is found in two allotropes: alpha-tin and beta-tin. Alpha-tin is a brittle, dull, powdery, semimetallic form of tin. It is made when very pure tin is cooled. Beta-tin is the normal shiny, soft, conductive, metallic form. It is made at higher temperatures. The decay of tin by turning from beta-tin to alpha-tin is called tin pest. Alpha-tin is not wanted in many places. When small amounts of other elements like antimony are added, the tin cannot change into alpha-tin. When alpha-tin is heated, it changes into beta-tin.
Tin can be hardened by adding antimony or copper, as well as some other elements. These also make it resistant to tin pest. Tin can also be made very shiny. Tin can make an alloy with copper called bronze.
Chemical properties
Tin resists many corrosive substances and is often used to protect other metals. Salt water and fresh water do not affect tin. It dissolves in strong acids to make tin salts. It reacts with some strong bases.
Chemical compounds
-
See also: Category:Tin compounds
Tin forms chemical compounds in two oxidation states: +2 and +4. +2 compounds are reducing agents. Some of them are colorless while others are colored. +4 compounds are more unreactive and act more covalent.
Tin burns in air to make tin(IV) oxide, which is white. Tin(IV) oxide dissolves in acids to make other tin(IV) compounds. Tin(IV) chloride is a colorless fuming liquid when anhydrous and a white solid when hydrated. It easily reacts with water to make tin(IV) oxide and an acid again.
Tin reacts with hydrohalic acids to make tin(II) halides. For example, tin(II) chloride is made when tin dissolves in hydrochloric acid. Tin(IV) halides are made when tin reacts with the halogens. Tin(IV) chloride is made when tin reacts with chlorine. Tin(II) sulfate is different as it does not oxidize to tin(IV) sulfate. Tin(II) oxide is a blue-black solid that burns in air to make tin(IV) oxide.
- +2 compounds
+2 compounds are reducing agents. They are about as common as +4 compounds. Some are colorless, while others are colored.
- Tin(II) bromide, yellowish solid
- Tin(II) chloride, white solid
- Tin(II) fluoride, white solid
- Tin(II) iodide, orange solid
- Tin(II) oxide, blue-black solid
- Tin(II) sulfate, white solid
- Tin(II) sulfide, dark brown solid
- +4 compounds
+4 compounds are unreactive. Some are colorless.
- Tin(IV) bromide, colorless solid
- Tin(IV) chloride, colorless liquid or solid
- Tin(IV) fluoride, colorless solid
- Tin(IV) iodide, orange solid
- Tin(IV) oxide, colorless solid
- Tin(IV) sulfide, gold-colored solid
Occurrence
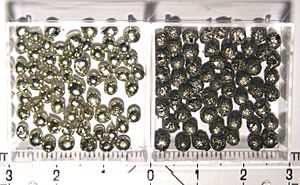
Tin is not found as a metal in the ground. It is normally in the form of cassiterite. Cassiterite is a mineral containg tin(IV) oxide. The cassiterite is normally found downstream of the cassiterite deposit when it is by a stream or river. Tin is also found in some complicated sulfide minerals.
Tin does not have any major job in the human body.
Preparation
Tin is made by heating cassiterite with carbon in a furnace. China is the biggest maker of tin.
History
People discovered tin long ago and used it with other metals. When copper and tin are mixed together, bronze is made. Bronze was important in the past, because it was one of the strongest metals available, which meant it was useful in weapons and tools. Bronze changed the world when it was first invented, starting the Bronze Age. People organized themselves more, because making tools from bronze was harder than making them from rock and wood like they did before.
Uses
Tin is used in solder. Solder used to contain a mixture of lead and tin. Now the lead is removed because of its toxicity.
Tin is also used to make pewter, which is mainly tin mixed with a small amount of copper and other metals. Babbitt metal also has tin in it. Tin is used to coat several metals, like lead and steel. Tin plated steel containers are used to store foods. The pipes on a pipe organ are made of tin. Tin foil was used before aluminium foil. Tin was one of the first superconductors to be found. Organotin compounds are more common than almost any other organometal compound. They are used in some PVC pipes to stop them from decaying. Organotin compounds are toxic, though.
Safety
Tin is not toxic, but tin compounds are very toxic to marine life. They are a little toxic to humans.
| Periodic table | |||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H | He | ||||||||||||||||||||||||||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||||||||||||||||||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||||||||||||||||||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | ||||||||||||||||||||||||
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | ||||||||||||||||||||||||
| Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | ||||||||||
| Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Uut | Fl | Uup | Lv | Uus | Uuo | ||||||||||
|
|||||||||||||||||||||||||||||||||||||||||
Images for kids
-
Ball-and-stick models of the structure of solid stannous chloride (SnCl2).
-
Sample of cassiterite, the main ore of tin
See also
 In Spanish: Estaño para niños
In Spanish: Estaño para niños