Transition state facts for kids
In a chemical reaction, the transition state is the point where there is a maximum value of energy. This energy is called the activation energy. When two or more molecules are mixed, they will hit each other. If they hit with enough energy to go through the transition state, they will react and form new molecules. At the transition state, new bonds are formed while the old ones are broken. In a graph or a drawing, the transition state is often marked with the double dagger ‡ symbol.
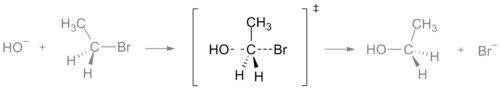
It is very difficult to study a transition state. This is because it is so high in energy that the molecules will stay in that form for a very short time, usually femtoseconds. It is important not to confuse transition states with intermediates. Intermediates are found at minimum points of energy, and they can live for a very long time. Like a transition state, however, an intermediate is between reagents and products of a reaction.
Studying transition states is very important to understand reaction mechanisms. There are theories and computer programs that can be used to calculate how the transition state looks like. This is a part of chemical kinetics.
See also
 In Spanish: Estado de transición para niños
In Spanish: Estado de transición para niños

