Zinc nitrate facts for kids
Quick facts for kids Zinc nitrate |
|
|---|---|
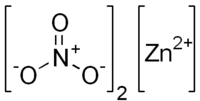 |
|
 |
|
| IUPAC name | Zinc nitrate |
| Identifiers | |
| CAS number | , 19154-63-3 (tetrahydrate) 10196-18-6 (hexahydrate) |
| PubChem | |
| EC number | 231-943-8 |
| RTECS number | ZH4772000 |
| SMILES | [N+](=O)([O-])[O-].[N+](=O)([O-])[O-].[Zn+2] |
|
InChI
InChI=1/2NO3.Zn/c2*2-1(3)4;/q2*-1;+2
|
|
| Properties | |
| Molecular formula | Zn(NO3)2 |
| Molar mass | 189.36 g/mol (anhydrous) 297.49 g/mol (hexahydrate) |
| Appearance | colorless, deliquescent crystals |
| Density | 2.065 g/cm3 (hexahydrate) |
| Melting point |
110 °C (anhydrous) |
| Boiling point |
~125 °C, decomp (hexahydrate) |
| Solubility in water | 327 g/100 mL, 40 °C (trihydrate) 184.3 g/100 ml, 20 °C (hexahydrate) |
| Solubility | very soluble in alcohol |
| Hazards | |
| MSDS | ICSC 1206 |
| EU Index | Not listed |
| Main hazards | Oxidant, may explode on heating |
| Flash point | Non-flammable |
| Related compounds | |
| Other anions | Zinc sulfate Zinc chloride |
| Other cations | Cadmium nitrate Mercury(II) nitrate |
| Related compounds | Copper(II) nitrate |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
Zinc nitrate is a chemical compound. Its chemical formula is Zn(NO3)2. It contains zinc and nitrate ions.
Contents
Properties
Zinc nitrate is a colorless solid. It dissolves in water. It reacts with bases to make zinc hydroxide. It reacts with sodium carbonate to make zinc carbonate.
Preparation
It can be made by reacting zinc metal or zinc oxide with nitric acid.
Uses
It can be used as a mordant. It is also used as a source of zinc ions.
Related pages
See also
 In Spanish: Nitrato de cinc para niños
In Spanish: Nitrato de cinc para niños

All content from Kiddle encyclopedia articles (including the article images and facts) can be freely used under Attribution-ShareAlike license, unless stated otherwise. Cite this article:
Zinc nitrate Facts for Kids. Kiddle Encyclopedia.
